Research Article
Anti-anxiety effects in mice following acute administration of Ficus Thonningii (wild fig)

Aduema W1*, Akunneh-Wariso C2, Ejiofo DC3 and Amah AK3
1Department of Medical Physiology, Pamo University of Medical Sciences, Port Harcourt, Rivers State, Nigeria
2Department of Human Physiology, Abia State University, Uturu, Abia State, Nigeria
3Department of Medical Physiology, Imo State University, Imo State, Nigeria
*Address for Correspondence: Aduema W, Department of Human Physiology, PAMO University of Medical Sciences, Port Harcourt, Rivers State, Nigeria, Tel: (+234)8038046678; Email: [email protected]
Dates: Submitted: 27 August 2018; Approved: 10 September 2018; Published: 11 September 2018
How to cite this article: Aduema W, Akunneh-Wariso C, Ejiofo DC, Amah AK. Anti-anxiety effects in mice following acute administration of Ficus Thonningii (wild fig). Insights Depress Anxiety. 2018; 2: 040-047. DOI: 10.29328/journal.ida.1001009
Copyright License: © 2018 Aduema W, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ficus thoningii; Swiss white mice; Light/dark transition box; anxiety
Abstract
The effect of acute administration of ethanol extract of F. thoningii on anxiety and fear in Swiss white mice was studied. 30 adult Swiss white mice of both sexes were randomly divided in to three groups of 10 mice each. Group1 served as the control and was administered normal saline only. Group 2 (low dose group) was administered 10mg/kg ethanol extract of the F. thoningii, while group 3 (high dose group) was given 20mg/kg of the same extract. All animals were allowed food and water ad libitum. Neurobehavioral parameter was assessed using the light/dark transition box. The analysis of variance (ANOVA) was used to test for variability within and among groups. Results were expressed as Mean ±SEM (standard error of the mean) and probability level p<0.05 was accepted as significant. The result showed that the frequency of transition in the light/dark transition box was significantly increased in the test groups (p<0.05; p<0.01).Similarly, the Light Box Duration was also significantly increased (p<0.01) in the low and high dose groups respectively. However, the Dark box duration was significantly decreased (p<0.05; p<0.01) in the low and high dose groups compared to control. This index showed a decreased level of anxiety and fear in the test groups. This was followed by a corresponding trend of decreased frequency of stretch attend posture and duration of freezing in the light/dark transition box (p<0.01; p<0.001) compared to the control. Summarily, acute administration of ethanol extract of F. thonningii causes calmness and sedation in moderate and high doses. It is therefore likely that it reduces aggression. If the result from this finding is extrapolated to humans, F. thoningii could be used to reduce anxiety disorders.
Introduction
The common wild Figure, Ficus thonningii, is one of the many fruit-bearing trees that have traditionally been used for treating diseases in Africa and beyond. The therapeutic properties of this tree are of paramount importance to traditional medicine. It is extensively used as a remedy for the treatment and cure of many diseases which include diarrhoea, gonorrhoea, vomiting, diabetes mellitus, respiratory infections, urinary tract infections, fever and mental illnesses [1.2]. Ficus thonningii is also widely used by nursing mothers to stimulate lactation (galactogogue) [3]. Despite its widespread use in ethnomedicinal systems, the safety and efficacy of Ficus Thonningii cannot be reliably guaranteed by the history and extent of its traditional use. F. thonningii is a multistemmed, evergreen or briefly deciduous tree with a dense, rounded to spreading crown mainly distributed in upland forests of tropical and sub-tropical Africa [4]. The tree grows at altitudes of between 1 000–2 500 m and it grows best in light, deep and well drained soils [4.5]. The leaves are alternate or whorled, mid-dark green and sub-glossy above whilst paler below. They can be rounded or tapering, 4.5-12 cm long, hairless or finely hairy with a prominent midrib [6]. The bark is usually smooth, pale to dark grey with vertical lenticels and the tree usually has aerial roots that hang from branches [4,6]. The fruits which are borne singly or in pairs are round, 10-20 mm in diameter, usually hairy and turn yellowish and rarely pink when ripe [7]. F. thonningii is a flowering tree that is pollinated by wasps which enjoy a symbiotic relationship and live in the syconium of its fruit 5 [4]. The tree can easily be propagated using seeds and cuttings [8]. Ficus thonningii has been described as a complex of taxa under which Ficus rokko, Ficus rhodesiaca, Ficus burkei, Ficus petersii, Ficus persicifolia and a number of other Ficus species are all lumped [7,9,10]. However, it is important to note that most of the trees grouped under the F. thonningii complex have the same name in the local African languages. The Shona name for F. rokko, F. natalensis, F. burkei and F. rhodesiaca is ‘Mutsamvi’ [6,10] and in Angola, ‘Mulemba’ is the local name given to F. burkei, F. psiliopoga and F. petersii [10]. More importantly, the traditional medicinal uses and value to the local people appears to be the same for these closely related variants. F. thonningii leaves are a good source of protein [11]. Protein content in F. thonningii leaves ranges between 18.7-20.5 g/100 g dry matter (DM) [11]. This makes them a useful dietary source of essential and non-essential amino acids. F. thonningii leaves therefore have the potential to be used to militate against protein deficiency diseases in children particularly in drought prone areas. The leaves are also good sources of micronutrients. They have an ash content of up to 17.34% w/w [12]. F. thonningii also has high levels of calcium, 180.05 mg/100 g dry weight [13] as compared to that in bovine milk (118 mg/100 ml). Potassium levels range between 0.91 and 1.25 g/100 g dry weight and magnesium levels between 260 and 357.2 mg/100 g dry weight [13,14] F. thonningii leaves have also been shown to contain a high crude fibre content which reaches up to 19.41 % w/w [11]. Crude fibre is important, particularly in ruminant nutrition, for the production of volatile fatty acids which account for about 80% of the metabolic energy requirements. Owing to its good nutrient profile, F. thonningii is used as forage for ruminants [14] and rabbits [11,12]. A comparative study done to evaluate the utilisation of F. thonningii and Mangifera indica (mango) leaves by rabbits showed that rabbits on F. thonningii leaves had a significantly higher average body mass compared to those on Mangifera indica leaves [12]. African ethnic groups have reported the use of F. thonningii in cuisine. The evergreen leaves are cooked as a vegetable by the Igede and Fulani of Nigeria [15], and the Senegalese [16]. The leaves are similarly used in Angola, Sudan, Benin and Ethiopia [8,17]. In addition to its widespread use as a food supplement and vegetable, F. thonningii is extensively used as medicine for treating disease, maintaining health and restoring tissue functioning and vitality. The broad pharmacological activity of F.thonningii can be seen from its application in the treatment of diverse infectious and non-infectious diseases. Its therapeutic potential can be credited to the presence of various phytochemicals that exhibit physiological effects.
F. thonningii contains different classes of secondary metabolites. These non-nutritive compounds, commonly known as phytochemicals, are produced by the plant for protection against biotic and abiotic stresses. They are generally responsible for the protective and disease preventive properties of many plants [18]. A survey of literature reveals that the main classes of phytochemicals isolated from F. Thonningii are: flavonoids, tannins, alkaloids, terpenoids and essential oils [2,19]. Therefore, the aim of the study was to investigate the anti-anxiety effects of Ficus. thonningii, using mice as experimental animals.
Materials and Methods
Animals
Thirty (30) Adult mice of both sexes weighing between 17-24g from animal room of the department of physiology, Abia state university, were used for this research work. These animals were kept in separate plastic cages in the animal room of Department of Physiology, Abia State University. They were fed with normal rodent chow and given water freely for two weeks to allow for acclimatization before the commencement of the experiment.
Animal treatment/Administration
The experimental mice which were 30 in number were divided into three (3) groups and each group contains ten mice. The animals in Group 1 (control group), received normal saline, while group 2 and 3 which were the low dose and high dose groups were given 10mg/kg and 20mg/kg of the extract each day. Prior before administration, the LD50 of the plant (ficus thonningii) was first established and the drug was administered via oral cannula for two weeks.
Ethical approval
I hereby declare that principle of Laboratory animal care was followed. All animals have been examined and approved by the appropriate ethics committee of the college of Medicine & Health Sciences of Abia State University, Uturu, Nigeria.
Experimental apparatus
The Light-Dark Box: The light-dark box test (LDB) is a popular animal model used in pharmacology to assay unconditioned anxiety responses in rodents [20].
Procedure
Rodents were placed in the light compartment of the apparatus and allowed to move around. Typically, rodents will move around the periphery of the compartment until they find the door. This process lasted between 7–12 seconds. All four paws must be placed into the opposite chamber to be considered as an entry. The following behaviors were recorded:
Transition: This is the period by which the animal walks in or out of the dark and light chambers with all four paws.
Period of dark box: This is the period of time in which the animal spends in the dark chamber.
Period of light box: This is the period of time in which the animal spends in the light chamber.
Line crossing: the number of times the animal crosses any particular line with all four limbs.
Stretch attends posture: frequency with which the animal demonstrates forward elongation of the head and shoulders.
Rearing: frequency with which the animal stands on its hind legs.
Grooming: frequency and duration of time the animal spent licking or scratching itself while stationary.
Experimental precaution
1. It was ensured that the measurement for drugs administration for low and high dose was appropriately measured.
2. During the experiment it was ensured that the time was accurately recorded.
3. During the experiment, it was ensured that we all sat 2meters away from the apparatus in order not to scare the mice.
4. Apparatus used for the experiment was cleaned using Cotton wool and 70% ethanol after each experiment.
Statistical analysis
Numerical data was compared using bar charts with error bars and presented as mean and standard deviations. Statistical significance was assumed at P≤0.05. Data collected were expressed as Mean ± SEM (standard error of mean), analysis of variance (ANOVA) and the Turkey’s multiple comparison tests (Post hoc test) were used for detailed analysis. “P” value less than 0.05, was considered statistically significant.
Results
Behaviors scored in the light/dark transition box test
Frequency of transition
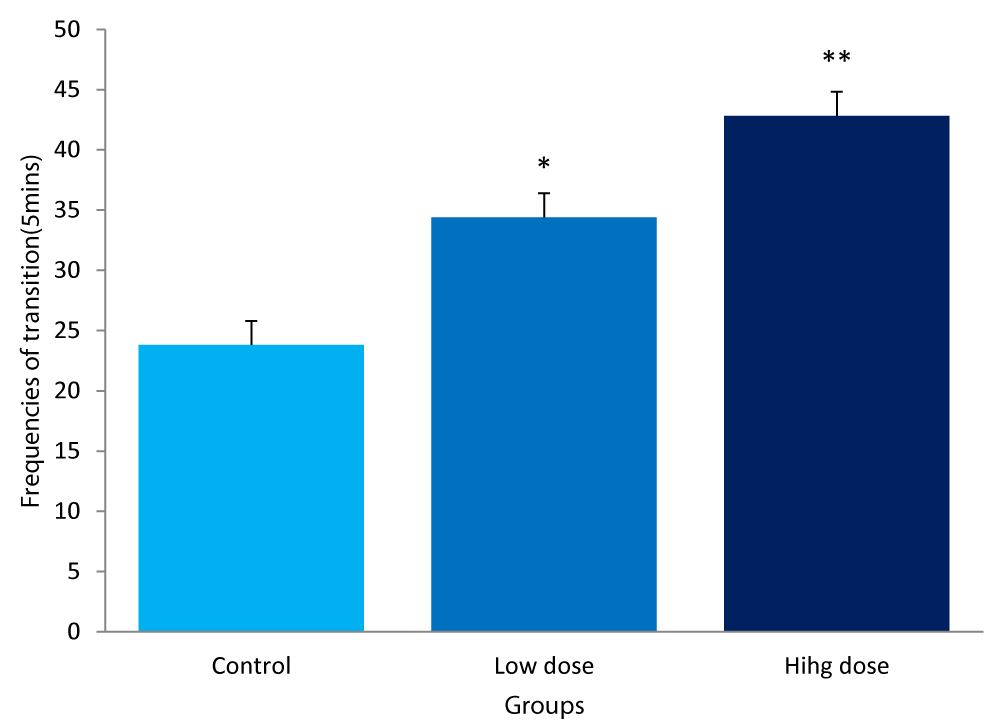
The values of the frequencies of transition following administration of F. thoningii among the experimental groups were:
Control group: 23.80± 5.89/5mins
Low dose: 34.80± 6.02/5mins
High dose: 42.80± 6.61/5mins
The frequencies of transition of the low dose group was significantly higher (P<0.05) compared to the control (Figure 1), whereas the value for the high dose group was also significantly higher (P<0.01) compared to control.
Figure 1: Comparison of frequency of transitions in the light/dark box between mice fed low &high dose F.thonningii and control. *-Significant at P<0.05 compared to control; **-Significant at P< 0.01 compared to control.
Light box duration
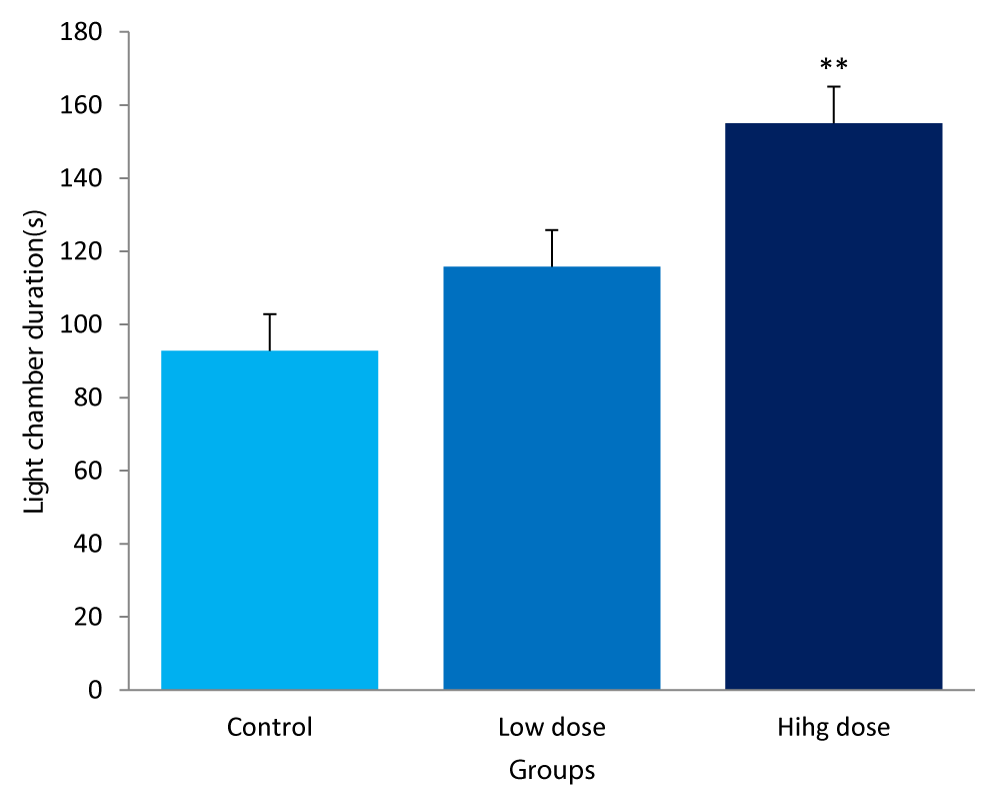
The values for the chamber duration in the light/dark transition box between mice fed low & high dose of F.thoningii and control diets are:
Control group: 92.80± 24.05secs
Low dose: 115.80± 8.58secs
High dose: 155.00± 40.87secs
The light chamber duration of the high dose group was significantly higher compared to control (P<0.01).However, the low dose group was statistically higher but not significant, when compared to the control (Figure 2).
Figure 2: Comparison of light chamber duration in the light/dark box between mice fed low &high dose F.thonningii and control. **-Significant at P< 0.01 compared to control
Dark Box duration
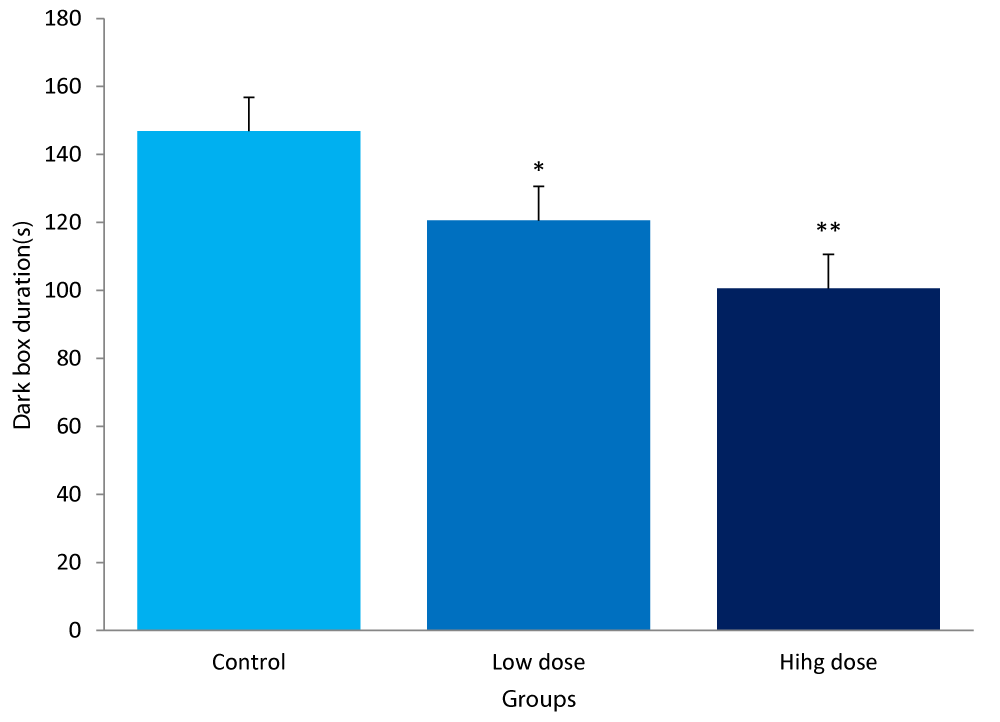
The values for the dark chamber duration in the light/dark transition box between mice fed low & high dose of F.thoningii and control diets are:
Control group: 146.80± 10.54secs
Low dose: 120.60± 17.14secs
High dose: 100.60± 16.78secs
The dark box duration in the light/dark transition box test for anxiety, for the low dose group was significantly lower compared to the control (P<0.05).The value for the high dose group was also significantly lower (P<0.01) compared to the control (Figure 3).
Figure 3: Comparison of dark box duration in the light/dark box between mice fed low &high dose F.thonningii and control. *-Significant at P<0.05 compared to control; **-Significant at P< 0.01 compared to control.
Frequency of stretch attend posture
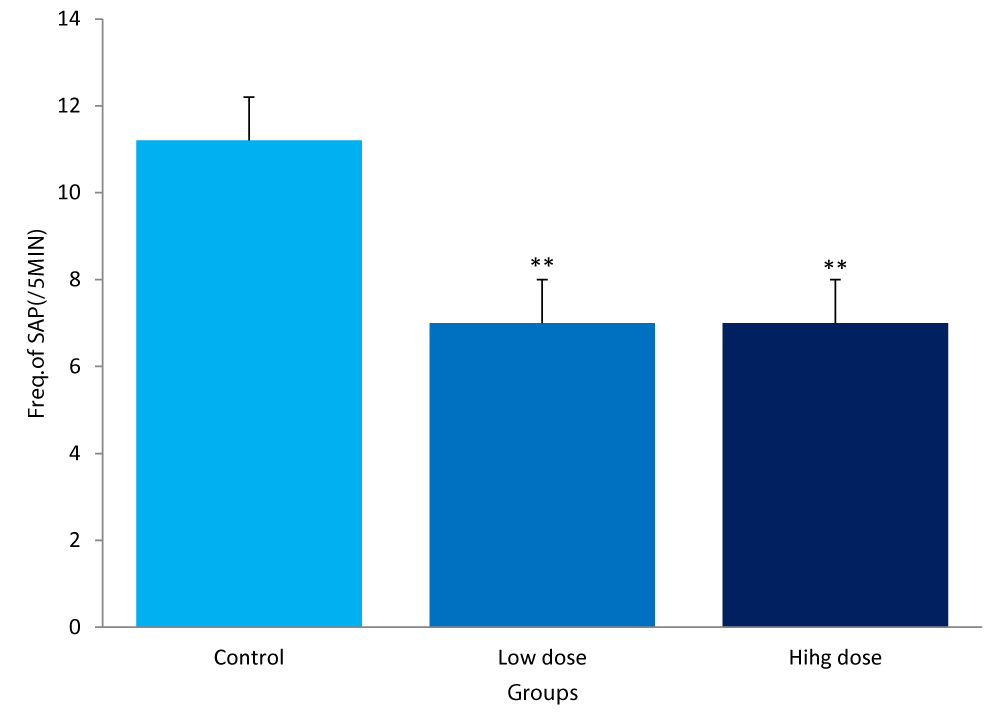
The frequencies of stretch attend posture between the groups of mice are:
Control group: 11.20± 1.64/5mins
Low dose: 7.00± 2.34/5mins
High dose: 7.00± 1.54/5mins
The frequencies of SAP for both the low and high dose groups were significantly lower (P<0.01) compared to the control (Figure 4).
Figure 4: Comparison of frequency of stretch attends posture in the light/dark box between mice fed low &high dose F.thonningii and control. **-Significant at P< 0.01 compared to control.
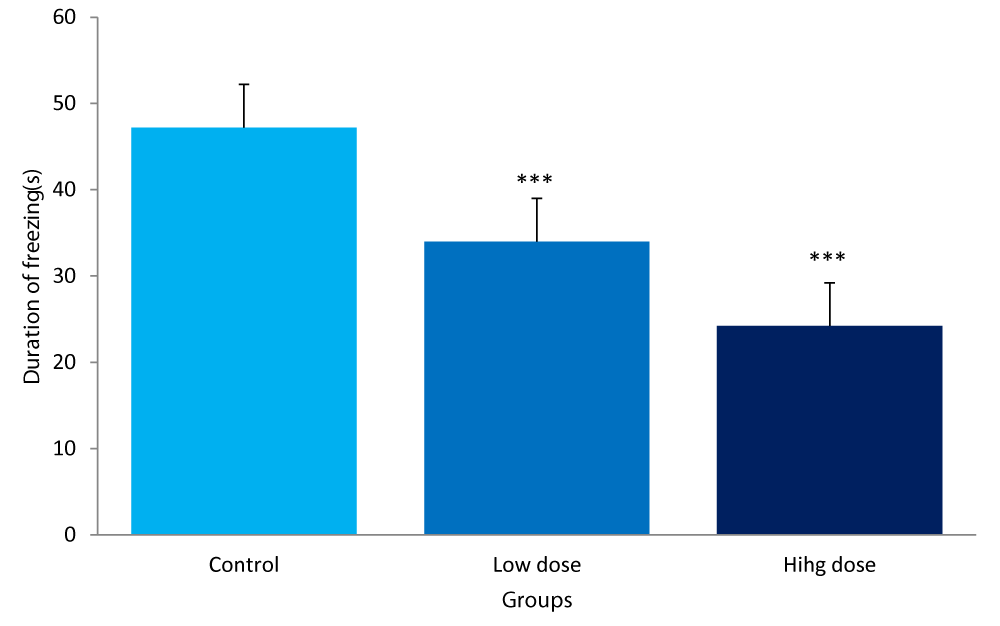
Freezing Duration
The duration of freezing between the groups of mice was:
Control group: 47.20± 2.58secs
Low dose: 34.00± 3.39secs
High dose: 24.20± 6.14secs
The durations of freezing for both the low and high dose groups were significantly lower (P<0.001) compared to the control (Figure 5).
Figure 5: Comparison of freezing duration in the light/dark box between mice fed low &high dose F.thonningii and control. ***-Significant at P< 0.001 compared to control.
Discussion
In the light-dark box, the frequency of transition for mice administered low and high dose of the extract of Ficus thonningii were significantly higher compared to control, indicating a decreased level of anxiety and fear related behavior. Stretch attend posture is a risk assessment behavior where rodents demonstrate forward elongation of the head and shoulder followed by retraction into its original position [21]. It is an anxiety behavior exhibited by rodents that are introduced into a novel environment. The frequencies of stretch attend posture (SAP) in the low and high dose groups were significantly lower compared to the control. Freezing duration in the light/dark transition box had a similar result like that of the stretch attend posture was obtained. During the light-dark transition box test, the chamber duration in the dark compartment of the box was significantly different in the three experimental groups when compared. However, the light chamber duration for the low and high dose groups were significantly higher when compared to the control. Rodents are known to spend less time in a well illuminated arena but this was contrary to what was observed in the treated groups of mice. Thus indicating a decreased level of anxiety in the treated groups. Anxiety and fear are basically controlled by the amydala. Osim [22] reported that electrical stimulation of this area was associated with the feeling of terror and fear in animals. It is possible that an unknown chemical constituents present in Ficus thonningii could be responsible for the anxiolytic effects observed in Ficus thonningii which inhibit the excitability of the amydala, thus reducing anxiety and fear like behaviors [23,24]. In conclusion, it is possible that moderate and high dosage of Ficus thonningii could show a remarkable difference in anxiety and fear related behaviors since it causes calmness and sedation. It is therefore likely that it reduces muscle tone and aggression and as such, could be used in the treatment and management of panic disorders.
Acknowledgement
We acknowledged Mr. and Mrs. B.A. Aduema, and Mr. Uchenna Azunna for their priceless support.
References
- Ajayi AO. Antimicrobial nature and use of some medicinal plants in Nigeria. African Journal of Biotechnology 2008; 7: 595-599. Ref.: https://goo.gl/yDL3F6
- Usman H, Abdulrahman FI, Usman A. Qualitative phytochemical screening and in vitro antimicrobial effects of methanol stem bark extract of Ficusthonningii (Moraceae) AJTCAM. 2009; 6: 289–295. Ref.: https://goo.gl/svYm8s
- Orwa C, Mutua A, Kindt R, Anthony S. Agroforestree database: a tree reference and selection guide. 2009; Ref.: https://goo.gl/vzYkXY
- Agroforestry Tree Database. 2011; Ref.: https://goo.gl/2j4GRd
- Hines DA, Eckman K. Indigenous multipurpose trees of Tanzania Uses and economic benefits for people. FAO Corporate document repository. 1993; Ref.: https://goo.gl/33kXX9
- Hyde MA, Wursten B. 2011 Flora of Zimbabwe. Ref.: https://goo.gl/vx4fLf
- Schmidt E, Lutter M, McCleland W. Trees and shrubs of Mpumalanga and Kruger National Park. Jacana, South Africa. 2002; 80 Ref.: https://goo.gl/SdzCy6
- Danthu P, Solovier P, Gaye A, Sarr A, Seck M, et al. Vegetative propagation of some West African Ficus species by cuttings. Agroforestry Systems 2002; 55: 57-63. Ref.: https://goo.gl/4b5KrW
- Beglinger C. Overview: cholecystokinin and eating. Current Opinion in Investigational Drugs. 2002; 3: 587-588. Ref.: https://goo.gl/knjoau
- Burrows J, Burrows S. Figureures of Southern and South-Central Africa, Umdaus Press. Hatfield, South Africa. 2003; 62-100. Ref.: https://goo.gl/vUQGcs
- Tegbe TSB, Adeyinka IA, Baye KD, Alawa JP. Evaluation of feeding graded levels of dried and milled Ficusthonningii leaves on growth performance, carcass characteristics and organ of weaner rabbits. Pakistan J Nutr 2006; 5: 548–550. Ref.: https://goo.gl/Ds8ErU
- Jansen O, Angenot L, Tits M, Nicolas JP, De Mol P, et al. Evaluation of 13 selected medicinal plants from Burkina Faso for their antiplasmodial properties. J Ethnophamacol. 2010; 13: 143–150. Ref.: https://goo.gl/VRiXrJ
- Titanji VPK, Zofou D, Ngemenya MN. The antimalarial potential of medicinal plants used for the treatment of malaria in Cameroonian folk medicine. AJTCAM. 2008; 5: 302–321. Ref.: https://goo.gl/BbX9Nm
- Bamikole MA, Ikhatua UJ. Nutritional evaluation of Ficus thonningii-Panicum maximum mixtures in West African dwarf goats. Nutrition and Food Science. 2010; 40: 280–288. Ref.: https://goo.gl/uCkQ83
- Igoli JO, Tor-Anyin TA, Usman SS, Oluma HOA, Igoli NP. Folk medicines of the Beneu valley of Nigeria. In: Singh VK, Govil JN, Hashim S, Sing G, 2002. Recent progress in Medicinal plants, Ethnomedicine and Pharmacognosy. Raleigh, NC: Science Technology Publishers. 2010; 327–338. Ref.: https://goo.gl/BGLmpi
- Mathieu G, Meissa D. Traditional leafy vegetables in Senegal. Diversity and medicinal uses. AJTCAM. 2007; 4: 469–475. Ref.: https://goo.gl/ZwTDmi
- Acamovic T, Brooker JD. Biochemistry of plant secondary metabolites and their effects in animals. Proceedings of the Nutrition Society. 2005; 64: 403-412. Ref.: https://goo.gl/hV7wrf
- Cowan MM. Plant products as antimicrobial agents. Clinical Microbiology Review 1999: 12: 564-582. Ref.: https://goo.gl/qe6xBg
- Ahur VM, Madubunyi I, Adenkola AY, Udem SC. The effect of acetyl acetate extract of Ficus thonningii (Blume) leaves on erythrocyte osmotic fragility and hematological parameters in acetaminophen-treated rats. Com Clin Pathol. 2010; 10: 1107–1111. Ref.:
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behaviour in genetically modified mice. Molecular Psychiatry. 2004; 9: 326-357. Ref.: https://goo.gl/4VUynB
- Nilson R. A qualitative and quantitative risk assessment of snuff dipping. Regulatory Toxicology and Pharmacology. 1998; 28: 1-16. Ref.: https://goo.gl/dmDPrA
- Osim EE. Neurophysiology. Calabar. University of Calabar Press. 2008; 24-27.
- Aduema W, Munach I, Akunneh-Wariso C. Effect of Ethanol Extract of Nymphaea lotus (Water Lily) on fear and anxiety Behavior in mice. Annals of Clinical and Laboratory Research. 2018; 6: 223. Ref.: https://goo.gl/jVfhML
- Adolphs R, Gasselin F, Buchanan TW, Tranel D, Scgyns P, et al. A Mechanism for impaired fear recognition after amygdala damage. Nature. 2005; 433: 68 -72. Ref.: https://goo.gl/DNRAek