Research Article
Vestibular-limbic relationships: Brain mapping

Paolo Gamba*
Department of Otolaryngology - Head and Neck Surgery Poliambulanza Foundation Hospital, via Leonida Bissolati, 25124 - Brescia, Italy*Address for Correspondence: Paolo Gamba, Department of Otolaryngology-Head and Neck Surgery Poliambulanza Foundation Hospital, via Leonida Bissolati, 25124-Brescia, Italy, Email: [email protected]
Dates: Submitted: 17 December 2016; Approved: 15 March 2018; Published: 16 March 2018
How to cite this article: Gamba P. Vestibular-limbic relationships: Brain mapping. Insights Depress Anxiety. 2018; 2: 007-013. DOI: 10.29328/journal.ida.1001006
Copyright License: © 2018 Gamba P. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Amygdala; White matter lesions; Chronic subjective dizziness; Anxiety
Abstract
Vestibular disorders and anxiety are closely related, probably because they share some neuronal pathways. Ageing and patient comorbidities are important facilitating factors, and multiple vascular risk factors could contribute to the onset of a vestibular syndrome called vascular vertigo. White matter lesions (WML) are often seen on magnetic resonance imaging (MRI) scans of elderly people and are related to various geriatric disorders, including dizziness. The cause of this correlation could be the disruption of neuronal networks that mediate higher vestibular cortical function. Numerous neuronal pathways link the vestibular network with limbic structures and the prefrontal cortex modulates anxiety through its connections to amygdala. The aim of the present work was to investigate the correlation between WML, amygdala and cognitive functions.
Introduction
The traditional classification of vestibular disorders is based on the anatomical site of the lesion and distinguishes between the peripheral and the central vestibular system [1]. This classification could not reflect the clinical syndrome, which may involve both the peripheral and the central vestibular system, leading to “higher vestibular function” disorders, since inputs from visual, vestibular and proprioceptive systems are integrated and elaborated through cognitive functions [2]. Numerous polysynaptic pathways link the vestibular network with limbic, hippocampal, cerebellar and cerebral cortex structures in order to mediate higher cognitive functions. The vestibular system provides the brain with sensory signals for postural and oculomotor control, as well as for spatial and body perception and cognition. The descending pathways from the cerebral cortex and the brain stem to the spinal interneurons and motor neurons must represent the instrument by which the brain steers movements of body, they are referred as the motor system. In earlier years, the motor system of the brain tended to be identified with the motor cortex. The corticospinal fibers are mainly derived from the sensorimotor areas and terminate predominantly contra laterally in the spinal gray. A striking parallel exists between the development of the corticomotoneuronal connections and the capacity to execute highly fractionated movements. Two critical functions of the corticospinal tract are important: first, the role of descending projections to the dorsal horn in the control of sensory afferent input, and second, the capacity of direct cortico-motoneuronal projections to support voluntary execution of skilled hand and finger movements. Therefore, any differences in the organization of corticospinal projections across species may well reflect differences in their functional roles. These movements are often particularly vulnerable to neurological disease, including stroke, cerebral palsy, movement disorders, spinal injury, and dizziness. Additionally, a central lesion, such as cerebellar infarctions, may mimic a peripheral disorder and could be misinterpreted [3]. Vestibular disorders lead to a significant degree of handicap and emotional disturbance for the patient. Recent epidemiological studies have reported a high prevalence of WMLs in the brains of elderly people. This study has investigated determinants of chronic and disabling cardiovascular, neurological, loco motor, and ophthalmologic diseases in the older population [4]. The prevalence and severity of WMLs increased with age. In addition, a history of stroke or myocardial infarction was significantly and independently associated with the presence of WMLs. Another European population-based study, examined randomly selected neurologically non diseased participants aged from 55 to 95 years (mean, 71.5 years) and found a WML prevalence rate of 39%. Increased age, silent brain infarction, and central cerebral atrophy were also significantly associated with WMLs [5]. WMLs were also significantly associated with age, smoking, lower education, and hypertension. Participants with psychiatric disorders such as depression or dementia have been reported to have a higher prevalence rate of WMLs than the general population. Thus, WMLs are extremely common in elderly people and they increase in prevalence with age, cardiovascular risk factors, dementia, and depression [6]. In the first days after an acute vestibular deficit, motor and spatial learning becomes very important to facilitate recovery of the balance function. In addition, new features emerge during this period, with implications on psyche hypointense on computed tomography are commonly referred to as white matter lesion (WML) [7]. Currently, WML are divided into periventricular white matter lesions, which are attached to the ventricular system, and deep white matter lesions, located in subcortical regions. MRI features of WML are correlated with dilatation of perivascular spaces, especially in the frontal and parietal subcortical white matter. Recently, a large meta-analysis of 46 observational studies demonstrated that WML are associated with greater risk of future stroke, dementia and mortality [8]. There is evidence that periventricular white matter lesions are particularly related to cognitive decline, whereas subcortical white matter lesions may be related to late onset depression. In a recent cohort study, the presence of thalamic lacunes was associated with poor global cognitive performance, low motor activity and poor executive function performance; moreover the presence of lacunes in the pallidum or putamen was associated with memory dysfunctions. Above all, deep brain infarcts have been associated with vascular cognitive impairment, including cognitive decline and dementia [9]. Although we know the association of WML, cognitive decline, aging, vascular risk factors, and actual dementia, the primary underlying mechanism of these processes is still unclear. WML are also associated to gait disturbance and dizziness [10]. In a group of older patients suffering from subjective and objective abnormalities of gait and balance of unknown cause, Baloh found a significant more severe subcortical WML on MRI when compared with an age- and sex-matched control group [11]. Additionally, a recent retrospective case analysis showed increased severity and frequency of WML in subjects with unexplained dizziness, suggesting that WML could contribute to the development of dizziness [12]. The cause of this relation between WML and vestibular disorders is still unclear and may involve several mechanisms. Lesions could interfere with the central processing of sensorimotor signals leading to impaired postural responses or may cause a disconnection syndrome involving vestibular or locomotor areas of the brain [13]. Indeed, the human brain contains multiple neuronal networks that serve motor and neurobehavioral functions such as visuospatial ability, complex cognition, and emotion. WML might disrupt higher vestibular cortical functions involved in these networks. WML disease could be especially critical later in life, because white matter volume might decline with age more than gray matter volume [14]. Our team systematically studied dizzy patients with MRI to map WML and to verify the correlation between WML and vestibular disorders, in particular vascular vertigo. Reported below are our recent clinical experience, neuroradiological features and outcomes in managing dizziness in elderly patients at the Poliambulanza Foundation Hospital of Brescia.
Patients and Methods
A total of 90 patients (53 females and 37 males), with a mean age of 75 years (range 59-89 years), were observed from January 2014 to January 2015. All patients underwent a clinical vestibular examination. Based on clinical suspicion and investigation results they also underwent 1.5 T cranial MRI. All patients selected for this study showed at least 1 WML on MRI, had been suffering from imbalance for a minimum period of one year and had a diagnosis of vascular vertigo based on at least 3 of the following vascular risk factors: cerebrovascular diseases; carotid disease; ischemic heart disease; diabetes mellitus; arterial hypertension; arteriopathy; family history of vascular diseases; smoking; alcohol consumption; obesity; fibrinogen >350 mg/dl; triglycerides >180 mg/dl; cholesterol >220 mg/dl. Patients with psychiatric or neurological diseases were excluded. Total pazients had been suffering from imbalance for a minimum period of one year and had a diagnosis of non-vertiginous dizziness or Chronic Subjective Dizziness (CSD) according to Ruckenstein and Staab [15]. There are 3 primary factors that describe CSD: persistent non-vertiginous dizziness that has occurred for 3 months or longer, hypersensitivity to either their motion, or motion of the visual surroundings, difficulty with precision visual tasks. The symptoms of CSD, included non-vertiginous dizziness and unsteadiness that was increased by a person’s own motion, exposure to environments with described are: space-motion discomfort and visual vertigo.
Results
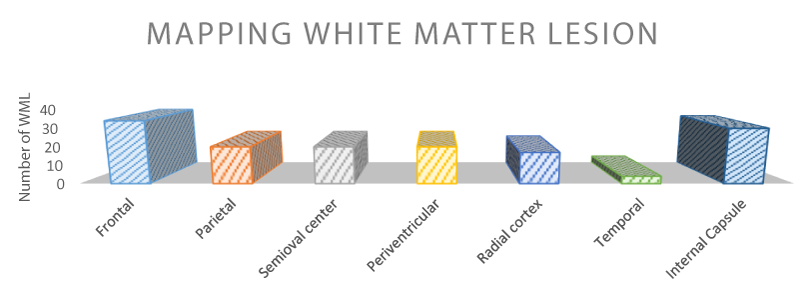
Vascular risk factors are outlined in table 1: 81% of patients had arterial hypertension, 67% had hypercholesterolemia and about 40% had hypertriglyceridemia, 15 patients out of 90 (17%) had already experienced cerebrovascular events. The table 2 shows the anatomical distribution of the WML. The most frequent sites are the frontal and around the limbic system areas (internal capsule), where lesions were present in 38 and 31 cases respectively. The prefrontal cortex (PFC), which is one of the last territories of the neocortex to develop, in evolution as well as ontogeny, its function is dedicated to the memory, planning, or execution of actions. The PFC can be subdivided in three major region: orbital and medial regions are involved in emotional behavior and lateral region, which is maximally developed he human, provides the cognitive support to the temporal organization of behavior, speech and reasoning. The orbital and medial PFC is the association cortex of the frontal lobe (in primates, it comprises areas 8-13,24,32,46,47) which is well connected with the braimstein and limbic formation, plays a major role in emotional behavior and the control of basic drives. A cardinal function of the lateral PFC is the temporal integration of information for the attainment of prospective behavioral goals. This function of temporal organization is served by several subordinate functions that are closely interwined (e.g., temporal integration, working memory). Much of the connectivity with subcortical structures is reciprocal. Especially well organized topologically are the connections between the PFC and the thalamus. The prefrontal connections with thalamus mediodorsal thalamic nucleus have been used as a criterion for identifying the PFC in a wide variety of species. This is the reason why the discussion of the operation of the PFC is here preceded by the placement of the PFC in a cortical connectionist map of cognitive representations. The study reveals widespread vestibular activations in the motor, visual, and somatosensory cortex; associative parietal cortex; prefrontal cortex; and thalamus and limbic structures. A regulatory control of frontal region over posterior systems for sensation and autonomic functions in a dense, interconnected network was suggested and associative relation within the right hemisphere were proposed to explain links among dizziness, nausea and negative emotions.
| Table 1: Vascular risk factors | ||
| Risk factors | N° patients/90 | % |
| Arterial hypertension | 73 | 81% |
| Hypercholesterolemia | 60 | 67% |
| Hypetriglycerydemia | 40 | 44% |
| Peripheral arterial disease | 38 | 42% |
| Cardiac disease | 35 | 39% |
| Diabetes | 30 | 33% |
| Cerebrovascular events | 15 | 17% |
| Total is >90 because patients can have more than one risk factor. | ||
Table 2: Mapping of White Matters Lesions. The table shows the frequency of mapped WML in the different anatomical regions in 90 patients suffering from vascular vertigo. The results showed that the most frequent WML sites were frontal and internal capsule areas. The total number of lesions is greater than the number of patients because one patient can have more than one lesion.
Discussion
The vestibular system is tightly connected to the limbic system, which in turn regulates emotions, homeostasis and storage of information. In particular, vestibular inputs are basic for the activity of the hippocampus and amygdala. The amygdala is another limbic component. There is a motivational circuit in the brain, centred on the amygdala that underlies animal and human emotion [16]. The amygdala, composed of three main nuclei and several nuclei, is connected with other areas of the brain throught two major bundles of fibres, the stria terminalis and the ventral amyddalofugal pathway. The amygdala is critically involved in acquisition and retaining of lasting memories of emotional experiences. It mediates the memory-modulating effects of adrenal stress hormones and several classes of neurotransmitter, which are selectively mediated by the basolateral complex of the amygdala (BLA) [17]. From the BLA of the amygdala, glutamatergic projections reach out to the anterior cingulate, prelimbic and infralimbic cortices of the medial prefrontal cortex. Disturbances in the medial prefrontal cortex-dopamine signaling affect the amygdala and may be responsible for emotional and cognitive disturbances [18]. The amygdala-driven signals can be correlated not only to the fusiform cortex and other distant areas such as anterior cingulate, superior temporal sulcus and lingual gyrus. The key role of the amygdala is in enabling and acquiring lasting memories of emotional and cognitive experiences. The amygdala is, particularly, more activated by anger and fear than happiness. In humans, amygdala lesions generate deficits in the recognition of fearful facial expressions, behavior and emotions. The combination of unpredictable vertigo attacks and accompanying severe vegetative reactions contributes to increase anxiety and fear. Recent reviews have considered that during the first vertigo attack 93% of patients have a fear of serious illness and 35% have panic [19,20]. Anxiety and fear increase in places with a rich visual surrounding and where movements require good head-eye coordination. Furthermore, anxiety is probably one of the most important causes preventing adaptation. The relation between anxiety and vestibular disorders could be explained by the fact that they share some neural pathways. This could lead to a vicious circle where dizziness and anxiety increase each other [21]. Dizziness affects about 30% of people over the age of 65. With increasing age, a progressive loss of function of the vestibular, visual and proprioceptive systems can lead to balance problems. Patient comorbidities also play an important role in aggravating the physiological degeneration of the balance system. For example, cardiovascular comorbidities are correlated to both peripheral and central vertigo [22]. The most important cardiovascular risk factors are: hypercholesterolemia, cardiac disease, diabetes, arterial hypertension and carotid atherosclerosis. Recent 4-years and 9-years follow-up studies also showed that subjects suffering from vertigo have a higher risk of stroke than the general population. Multiple vascular risk factors could contribute to the onset of a vestibular syndrome called vascular vertigo due to its vascular etiology [23]. The areas in cerebral white matter that appear hyperintense on T2-weighted Magnetic Resonance Imaging (MRI) and hypointense on computed tomography are commonly referred to as white matter lesion (WML) [24,25]. WML are attributed to degenerative changes of small vessels and of long penetrating arteries and are implicated in the pathogenesis of cognitive decline and dementia. Currently, WML are divided into periventricular white matter lesions, which are attached to the ventricular system, and deep white matter lesions, located in subcortical regions. WML are often seen on MRI scans of elderly people and are related to various geriatric disorders, including cerebrovascular diseases, cardiovascular diseases, dementia, and psychiatric disorders. MRI features of WML are correlated with dilatation of perivascular spaces, especially in the frontal and parietal subcortical white matter [26]. The left amygdala enhances responses to fearful faces more than happy ones and increases the intensity of fear [27]. However, a PET imaging study proposed a decrease in right amygdala activity under conditions of social anxiety induced by a mental arithmetic task. Fear-related facial modulations driven by amygdala signals may implicate not only the fusiform cortex (e.g. V1) and other distant regions involved in social, cognitive or somatic responses (e.g. superior temporal sulcus, cingulate or parietal areas) [28,29]. Normally, visual inputs reach the visual cortex through the thalamus. However, in instances of emotional visual inputs, direct or indirect emergency, activation of the amygdala with long-term potentiation (LTP) and the projection into hypothalamus and entorhinal cortex occurs. It has not yet been demonstrated but it is likely that rotational vertigo is a type of visual input with a component of emotional distress and fear that can activate the amygdala and related circuits of memory [30]. As seen in table 2, we found a high prevalence of frontal cortex and limbic structures WML in patients suffering from vascular vertigo. Frontal and prefrontal WML could be associated with both vestibular disorders and anxiety. In recent years, many authors have proposed the existence of a “vestibular cortex”. In humans, galvanic and caloric vestibular stimulation activates several frontal cortical regions [31-33]. Furthermore, studies on animals have focused on structures that receive vestibular inputs and are located in frontal, temporal and parietal cortex. Acute lesions of these areas, for example after middle cerebral artery infarcts, cause tilts of perceived vertical, body lateropulsion and rotational vertigo. The vestibular cortex intimately interacts with the visual cortex and numerous neuronal pathways link the vestibular network with limbic and hippocampal structures [34,35]. These structures are involved in the fear network model, which exhibits aberrant activation patterns in a variety of anxiety disorders. In particular, the prefrontal cortex modulates anxiety and other emotional behaviors through its connections to amygdala [36,37]. A recent study found alterations in frontal WM and WM around the frontal lobe in patients with panic disorders [38,39]. These neuronal connections with limbic structures could also explain abnormal levels of anxiety observed in patients suffering from vestibular deficit. Based on the consideration that WML and chronic subjective dizziness could have a vascular pathophysiology, the pharmacological intervention to improve vascular homeostasis could relieve symptoms of anxiety.
Conclusions
Vestibular disorders, ageing, multiple vascular risk and anxiety are intimately related and this relationship could be explained by the fact that they have some common neural pathways. The correlation could be further supported by the high prevalence of frontal WML among people with vestibular disorders and anxiety. Cardiovascular factors could have a pathogenetic role in WML as well as in vestibular disorders and in aggravating symptoms of both diseases. Our personal experience with patients suffering from vascular vertigo confirmed these correlations between WML, amygdala and cognitive functions and evaluate the possibility to employ a pharmacological (non-psychotropic) therapy with the purpose to avoid the chronic phase of the anxiety and fear symptoms in these clinically complex patients.
References
- Brandt T, Strupp M, Dieterich M. Towards a concept of disorders of "higher vestibular function". Front Integr Neurosci. 2014; 8: 47. Ref.: https://goo.gl/Fy1fNX
- Teggi R, Caldirola D, Perna G, Bellodi L, Bussi M. Vestibular testing in patient with panic disorders and chronic dizziness. Acta Otorhinolaryngol Ital. 2007; 27: 243-247. Ref.: https://goo.gl/grZHWL
- Simon NM, Pollack MH, Tuby KS, Stern TA. Dizziness and panic disorders: a review of the association between vestibular dysfunction and anxiety. Ann Clin Psychiatry. 1998; 10: 75-80. Ref.: https://goo.gl/F6gPwp
- Lindgren A, Roijer A, Rudling O, Norrving B, Larsson EM, et al. Cerebral lesions on magnetic resonance imaging, heart disease, and vascular risk factors in subjects without stroke. A population-based study. Stroke. 1994; 25: 929-934. Ref.: https://goo.gl/tJKA85
- Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994; 25: 318-327. Ref.: https://goo.gl/VoxUqq
- Longstreth WT Jr., Manolio TA, Arnold A, Burke GL, Bryan N, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996; 27: 1274-1282. Ref.: https://goo.gl/2qiko2
- Liao D, Cooper L, Cai J, Toole J, Bryan N, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. 1997; 16: 149-162. Ref.: https://goo.gl/GbSaHB
- Guidetti G. The role of cognitive processes in vestibular disorders. Hearing, Balance and Communication. 2013; 11: 3-35. Ref.: https://goo.gl/7GMKNy
- Monzani D, Casolari L, Guidetti G, Rigatelli M. Psychological distress and disability in patient with vertigo. J Psychosom Res. 2001; 50: 319-323. Ref.: https://goo.gl/XbhjoH
- Carmeli E. Anxiety in the Elderly Can be a Vestibular Problem. Front Public Health. 2015; 3: 216. Ref.: https://goo.gl/1EQyie
- Colledge N, Lewis S, Mead G, Sellar R, Wardlaw J, et al. Magnetic resonance brain imaging in people with dizziness: a comparison with non-dizzy people. J Neurol Neurosurg Psychiatry. 2002; 72: 587-589. Ref.: https://goo.gl/vGgknW
- Gufoni M, Guidetti G, Nuti D, Pagnini P, Vicini C, et al. The relationship between cognitive impairment, anxiety-depression symptoms and balance and spatial orientation complaints in the elderly. Acta Otorhinolaryngol Ital. 2005; 25: 12-21. Ref.: https://goo.gl/JTDqMe
- Guidetti G. La terapia della vertigine vascolare nella pratica ambulatoriale: esperienza multicentrica (Studio VascVert). Otorinolaringol. 2005; 55: 237-246.
- Maillard P, Fletcher E, Harvey D, Carmichael O, Reed B, et al. White matter hyperintensity penumbra. Stroke. 2011; 42: 1917-1922. Ref.: https://goo.gl/j1EDn6
- Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry. 2008; 15: 273-280. Ref.: https://goo.gl/QDQDcm
- Ruckenstein MJ, Staab JP. Chronic Subjective Dizziness. Otolaryngol Clin N Am. 2009; 42: 71-77. Ref.: https://goo.gl/5B3z4p
- Ott A, Breteler MM, van Harskamp F, Stijnen T, Hofman A. Incidence and risk of dementia. The Rotterdam study. Am J Epidemiol. 1998; 147: 574-580. Ref.: https://goo.gl/ceq4Uf
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003; 348: 1215-1222. Ref.: https://goo.gl/TpVogx
- Matsusue E, Sugihara S, Fujii S, Ohama E, Kinoshita T, et al. White matter changes in elderly people: MR-pathologic correlations. Magn Reson Med Sci. 2006; 5: 99-104. Ref.: https://goo.gl/wHg1Mk
- de Leeuw FE, de Groot JC, Bots ML, Witteman JC, Oudkerk M, et al. Carotid atherosclerosis and cerebral white matter lesions in a population based magnetic resonance imaging study. J Neurol. 2000; 247: 291-296. Ref.: https://goo.gl/fUkPZU
- Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010; 341: c3666. Ref.: https://goo.gl/Qp2RGN
- Benisty S, Gouw AA, Porcher R, Madureira S, Hernandez K, et al. Location of lacunar infarcts correlates with cognition in a sample of non-disabled subjects with age-related white-matter changes: the LADIS study. J Neurol Neurosurg Psychiatry. 2009; 80: 478-483. Ref.: https://goo.gl/S2SEsn
- Gong L, Liu XY, Fang M. Recent progress on small vessel disease with cognitive impairment. Int J Clin Exp Med. 2015; 8: 7701-7709. Ref.: https://goo.gl/e1bG6u
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013; 12: 822-838. Ref.: https://goo.gl/hZK6GF
- Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch Neurol. 1995; 52: 970-974. Ref.: https://goo.gl/LcQnm3
- Ahmad H, Cerchiai N, Mancuso M, Casani AP, Bronstein AM. Are white matter abnormalities associated with "unexplained dizziness"?. J Neurol Sci. 2015; 358: 428-431. Ref.: https://goo.gl/Wt2Ehk
- Mattana P, Mannello F, Ferrari P, Augus GB. Vascular pathologies and inflammation: the anti-inflammatory properties of Sulodexide. J Vasc Endovasc Surg. 2012; 19: 1-7. Ref.: https://goo.gl/qJzAzo
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neuroaci. 2004; 27: 1-28. Ref.: https://goo.gl/9RQcLA
- Fioresco SB, Tse MT. Dopaminerg regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci. 2007; 27: 2045-2057. Ref.: https://goo.gl/wm4T9z
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressionsand complex IAPS pictures: common and differential networks. Neuroimage. 2006; 31: 906-919. Ref.: https://goo.gl/uxJh88
- Doyère V, Debiec J, Monfils MH, Schafe GE, Le Doux JE. Synapse-specific reconsolidation of distinct fear memories in the latral amygdala. Nat Neurosci. 2007; 10: 4-6. Ref.: https://goo.gl/qBHEJ4
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000; 406: 722-726. Ref.: https://goo.gl/NDs91n
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004; 306: 2061. Ref.: https://goo.gl/sHPkvQ
- Kilts CD, Egan G, Gideon DA, Ely TD, Hoffman JM. Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. Neuroimage. 2003; 18: 156-168. Ref.: https://goo.gl/i95CLW
- Morrys JS, Friston Kj, Buchel C, Frith CD, Young AW, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998; 121: 47-57. Ref.: https://goo.gl/pGk9gK
- Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, et al. The neuronal correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006; 31: 2243-2253. Ref.: https://goo.gl/eDs5jn
- Brandt T, Dieterich M. The vestibular cortex. Its locations, functions, and disorders. Ann N Y Acad Sci. 1999; 871: 293-312. Ref.: https://goo.gl/njvVVz
- Marcelli V, Esposito F, Aragri A, Furia T, Riccardi P, et al. Spatio-temporal pattern of vestibular information processing after brief caloric stimulation. Eur J Radiol. 2009; 70: 312-316. Ref.: https://goo.gl/dK14o6
- Kim B, Kim JH, Kim MK, Lee KS, Kim Y, et al. Frontal white matter alterations in short-term medicated panic disorder patients without comorbid conditions: a diffusion tensor imaging study. PLoS One. 2014; 9: e95279. Ref.: https://goo.gl/LbpnNr